India granted approval to its first DNA based vaccine against the coronavirus. This is the world’s first DNA plasmid Covid-10 vaccine, developed by Ahmedabad-based pharmaceutical firm Zydus Cadila or Cadila Healthcare. ZyCoV-D is a three-dose Covid-19 vaccine that will be administered to people 12 years and above. The world’s second-worst hit nation granted approval to this vaccine for emergency use. Here’s what you must know.
World’s First DNA Plasmid Covid-19 Vaccine Gets Approval For Emergency Use In India
India seeks to bolster its immunisation drive to prevent a possible third wave of Covid infections. And for this, the country has approved ZyCoV-D developed by Zydus Cadila. This is the second homegrown vaccine authorised for emergency use after Hyderabad based Bharat Biotech’s Covaxin. Zydus Cadila earlier revealed that they can launch the 3-dose vaccine within 2 months of receiving approval. The three-dose shift has reported 67% efficacy against the symptomatic clinical trials in July. Cadila claims ZyCoV-D works against newer strains including the highly contagious Delta variant. This is the world’s first and India’s indigenously developed DNA based vaccine for Covid-19.
https://twitter.com/ZydusUniverse/status/1428735046190440455?ref_src=twsrc%5Egoogle%7Ctwcamp%5Eserp%7Ctwgr%5Etweet
Also Read: Sikkim To Welcome Tourists With One COVID-19 Vaccine Dose; RT-PCR Test Mandatory
Is Sputnik V Approved For International Travel? Everything You Need To Know
Experts are considering the Sputnik V vaccine developed Gamaleya Research Institute of Epidemiology and Microbiology, Russia as one of the best vaccines to boost immunity against COVID-19. The vaccine, which is claimed to be around 92% effective against the virus, has to be taken in two doses, with a gap of 21 days. Unlike Covaxin and Covisheid, Sputnik uses two different formulas for both its jabs to boost the immune system. But is Sputnik V approved yet for international travel? Read on to know the details.

Also Read: Vaccine Passports: Will You Need One The Next Time You Travel?
Is Sputnik V Approved For Getting A Vaccine Passport?
The Russian vaccine got approval from the Subject expert committee (SEC) for Emergency Use Authorisation (EUA). It is the third COVID-19 vaccine to get clearance in India. But will it be applicable for getting a vaccine passport? The European Union said it will only accept the Pfizer, Moderna, and Johnson & Johnson vaccines for its vaccine certificate plans as of now. This means that if you have not taken one of these vaccines, you may not be able to travel to popular European tourist destinations such as Portugal, Croatia, Spain, and Cyprus. However, as per reports, a few EU countries are planning to accept Russia’s Sputnik V vaccine soon.
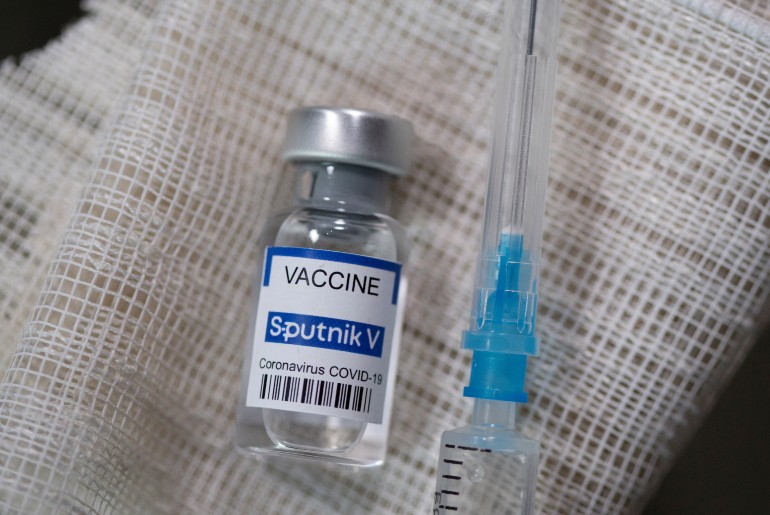
According to The Guardian, Thomas Mertens, the head of Germany’s standing commission on vaccination, said that Sputnik V is “a good vaccine that will presumably also be approved in the EU at some point”. Mertens told Rheinische Post, “Russian scientists are very experienced in vaccines.” Meanwhile, if you get shots of Covishield, you will be eligible to travel to other countries.
Also Read: Vaccinated With Covaxin? Your International Travel Plans May Get Hampered As Vaccine Not On WHO List
Sputnik V Vaccines In India
India has started importing Sputnik V vaccines and as per the latest reports, Apollo Group of Hospitals has announced that it will start administering the vaccine across its hospitals in India from the second week of June. The estimated price will be ₹1,195 per dose. According to an NDTV report, an official of the Apollo Group said, “We will be charging ₹ 995 for the vaccine and ₹ 200 would be administration charges.” Delhi Chief Minister Arvind Kejriwal has said the manufacturers of the jab have confirmed to send a consignment to Delhi after June 20.
First Published: August 21, 2021 10:44 AM